On Pills, Africa is yet to Scratch the Surface
By Muoki Musila
A Bitter Pill to Swallow
The COVID-19 vaccine gap exposed both opportunities and challenges for pharma in African countries with unpreparedness being a bitter pill to swallow for most nations and the private sector. This perhaps underlines the decision of the African Continental Free Trade Area Secretariat (AfCFTA) and the World Economic Forum (WEF) to consider the industry as part of other three that will drive Africa’s economic resurgence with the right investment. Africa’s pharma industry has various challenges, including a lack of local manufacturing, insufficient robust healthcare systems, weak regulatory systems, unethical business practices, sneaky local partners, and trade barriers that warrant urgent attention.
The industry is the worst-performing in the global value chains. Thus, the AfCFTA is strategically positioned to help enhance internal trade and supply routes to boot pharma trade across borders in Africa in addition to streamlining processes and harmonizing regulations that derail the progress. While players in the industry learned a valuable lesson from the COVID-19 scare, a great deal of work is needed to create an ideal environment for local production while strengthening healthcare systems.
A Challenged Industry Yet…
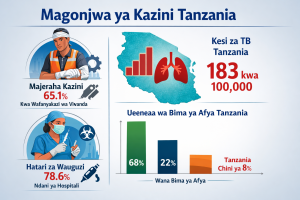
Lessons from the past show that Africa is extremely vulnerable with a poorly constituted and performing pharma industry underlining the need to create its healthcare ecosystems to help it measure up. Currently, Africa meets only 1% of its demand for essential medicine through domestic production. Kenya, the rapidly growing pharma industry heavyweight supplies 50% of the COMESA region’s demand while importing over 70% of the drugs used in its public hospitals. On one hand, this offers the much-anticipated trade potential for export into the African market while questioning the laxity of other AfCFTA partner states in facilitating investment into the sector.
“…removal of regulatory barriers that prevent patient access to critical medicine and the creation of a single African pharmaceutical market will incentivize investment and boost the continent’s capacity to manufacture 60% of vaccines needed locally…”
Notably, the sector, even in thriving sectors such as Kenya has to deal with stringent and expensive processes, including WHO prequalification on quality, safety, and efficacy of drugs further hindering the industry growth. However, considering cases such as recalled antifungal drugs made in Kenya by Rwanda, unethical business practices pose a challenge for African markets to compete with the rest of the world. Thus, in addition to dealing with poor standards in the industry, AfCFTA has its work cut out in dealing with burdensome prequalification compliance requirements that are also costly.
Whoever wants to bully Africa’s pharma industry will considering that most of the continent’s public health system relies on donor funds with hefty demands and conditions often favoring ‘big pharma’. With coordinated continental efforts, particularly around regional health security mechanisms and public-private partnerships, Africa has an adequate population to support trade and the growth of the industry. It is imperative that the private sector, governments, and trade blocs work together to achieve the security of supply boosting trade in pharma and investment in manufacturing capacities.
 The AfCFTA anticipates that the removal of regulatory barriers that prevent patient access to critical medicine and the creation of a single African pharmaceutical market will incentivize investment and boost the continent’s capacity to manufacture 60% of vaccines needed locally by 2040. Even with this, current Africa lacks a coherent single African market for the sector with manufactures required to conform to over 54 different regulatory agencies and meet local standards which elude desired economies of scale. The AfCFTA therefore has its work cut out in eliminating the individualized standards for players to capitalize on the African pharma market beyond existing borders.
The AfCFTA anticipates that the removal of regulatory barriers that prevent patient access to critical medicine and the creation of a single African pharmaceutical market will incentivize investment and boost the continent’s capacity to manufacture 60% of vaccines needed locally by 2040. Even with this, current Africa lacks a coherent single African market for the sector with manufactures required to conform to over 54 different regulatory agencies and meet local standards which elude desired economies of scale. The AfCFTA therefore has its work cut out in eliminating the individualized standards for players to capitalize on the African pharma market beyond existing borders.
Key Policy Imperatives
In addition to learning from the global markets, policymakers and the AfCFTA must prioritize learning from thriving African markets such as Kenya and South Africa, while fixing existing gaps that limit the markets. However, as is with the action plan by the AfCFTA and WEF on engaging the private sector, there is a need to increasingly put in place financing mechanisms for the value chains for low-interest capital and regional and continental private sector investment. Partnering with local companies to manufacture for the African market represents a feasible business opportunity for investors.
Being a highly capital-intensive industry, ethics and compliance are significant challenges for the industry with local manufacturers associated with counterfeiting and non-compliance with applicable local and international laws. Coordinated efforts are thus required, as with the harmonization of regulations and standards, to create a stable business environment where business ethics and the rule of law are respected.
The Big Pharma, representing globally dominant companies, have been associated with adopting contracts that make it hard for African local manufacturers to compete effectively within the continent. This is a room for African governments to prioritize incentives for local innovation and entrepreneurship in the sector through intellectual protection and enforcement for thriving intra-Africa trade. Moreover, players have to give the development and retention of local skilled workforces a chance to aid in the growth and development of the sector. With a sustainable and well-funded research and development ecosystems and efforts for new product development, the current brain drains to Europe and beyond can be effectively reduced.
An imperative for cross-border growth is the proliferation of current players within strategic manufacturing hubs within the continent which is pegged on harmonized and appropriate regulatory standards. The question is therefore whether the AfCFTA, private players, and member states can work out a mechanism for voluntary collaboration on technology transfer and best practices for the holistic growth of the industry. Further, would the intended regulatory approach allow for timely product registration and improved harmonization and reliance practices for improved supply chains for continental trade in pharma?
Support for Intra-Trade
The local market, a targeted market of 1.3 billion people, holds immense opportunities for domestic consumption of Africa’s pharma produce. However, there is a need for continued engagement and advocacy to support the strengthening of regulatory systems and convergence through the AfCFTA and other African bodies. Enabling and facilitating trade, such as through the implementation of the AfCFTA and regional trade protocols, to eliminate barriers and foster internationally viable supply chain security should be a priority.
Moreover, players, including individuals, the civil society, and governments are called upon to support the AfCFTA in fostering partnerships, driving investments, and countering illicit trade for improved business ethics and integrity in the sector. Ultimately, the free trade area has to advance engagements with the private sector and policy-makers on policy implementation in supporting regional value chains and encouraging growth of the pharmaceutical sector.
Muoki Musila is an Kenyan based economist. These are the writer’s own opinions and do not necessarily reflect the viewpoints of Liberty Sparks. Do you want to publish in this space? Contact our editors at [email protected] for further clarification.